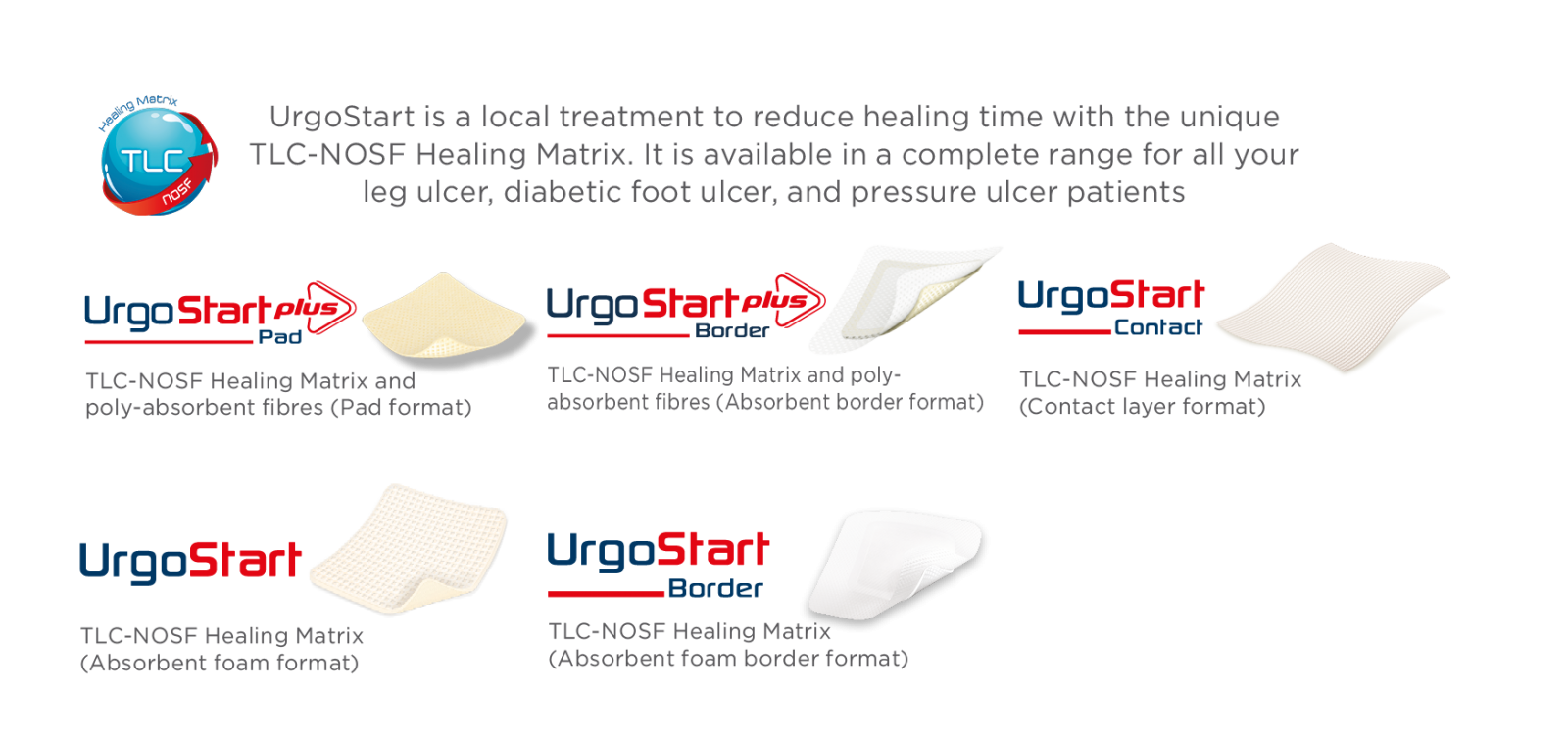
UrgoStart Plus treatment range is indicated at any healing stage for all leg
ulcers, diabetic foot ulcers, pressure ulcers and longstanding acute wounds. The TLC-NOSF Healing Matrix helps to clean and close the wound simultaneously, and returns the patient to healing even sooner.
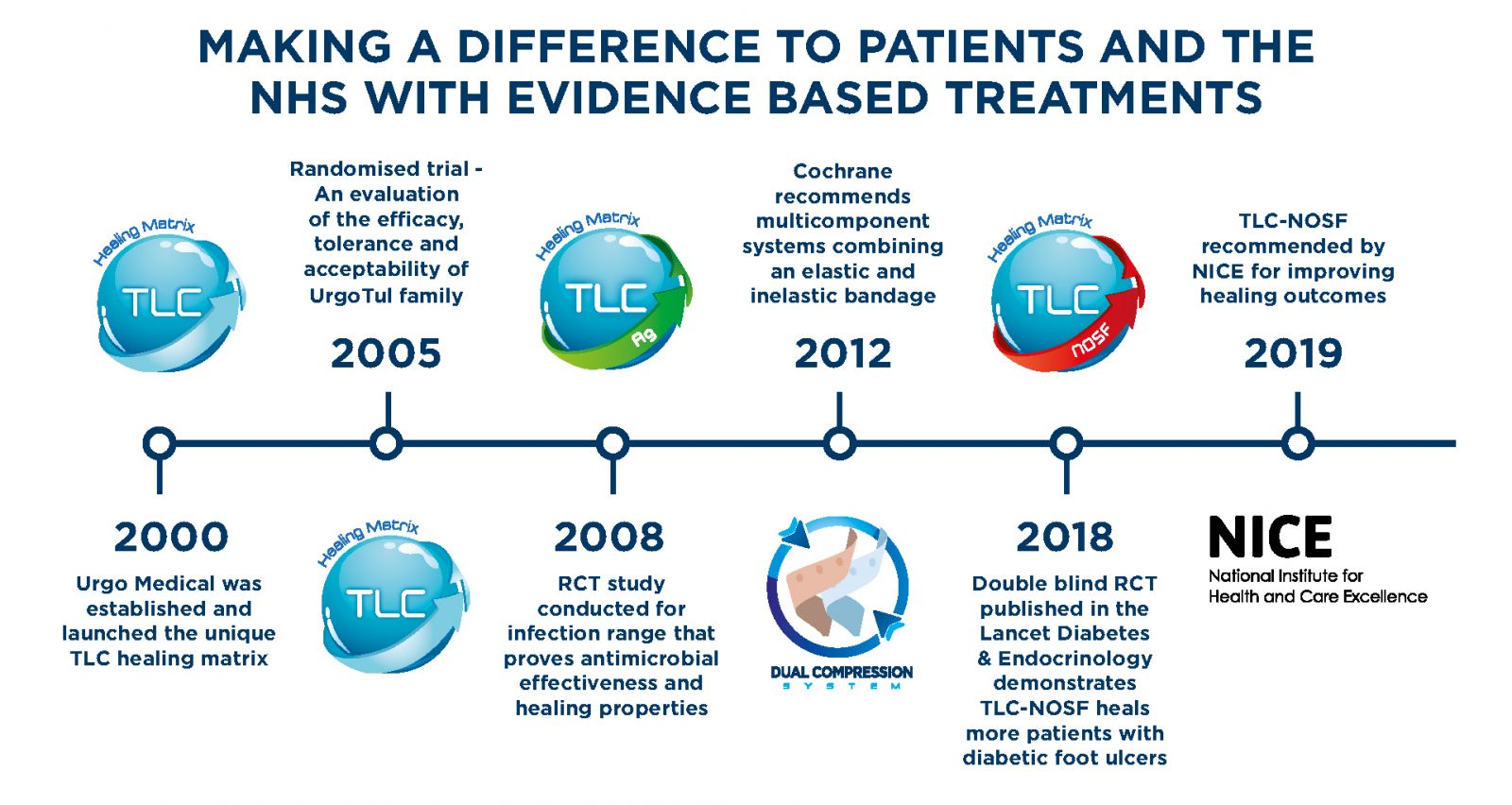
UrgoStart Plus treatment range is recommended by NICE for the treatment of venous leg ulcers and diabetic foot ulcers1, and is suitable for simple wounds
UrgoStart Plus treatment is the only treatment that is clinically proven to reduce healing time2-5
The sooner UrgoStart Plus treatment range is initiated the better the healing outcomes for patients6