References
Beitz AJ, Newman A, Kahn AR et al (2004) A polymeric membrane dressing with antinociceptive properties: analysis with a rodent model of stab wound secondary hyperalgesia. J Pain 5(1): 38–47
Benskin LL (2016) Polymeric membrane dressings for topical wound management of patients with infected wounds in a challenging environment: A protocol with 3 case examples. Ostomy Wound Manage 62(5): 42–50
Bode C, Woodman H (2008) Two novel treatments for the prevention and treatment of radiation induced moist desquamation. Poster presentation
Burd A, Kwok CH, Hung SC, et al (2007) A comparative study of the cytotoxicity of silver based dressings in monolayer cell, tissue explant, and animal models. Wound Repair Regen 15(1): 94-104
Cutting K, Gefen A (2019) PolyMem® and countering inflammation made easy. Wounds International. Available online: www.woundsinternational.com
Cutting KF, Vowden P, and Wiegand C (2015) Wound inflammation and the role of a multifunctional polymeric dressing. Wounds International 6(2): 41–6
De Leon J, Bohn GA, DiDomenico L, et al (2016) Wound care centers: Critical thinking and treatment strategies for wounds. WOUNDS 28(10): 3-24
Denyer J (2015) Six years’ experience of PolyMem® dressings used on children with epidermolysis bullosa (EB). Poster presentation. DEBRA International conference, 24 - 26 September. London, UK
Denyer J, Agathangelou C, White R, Ousey K, HariKrishna R (2015) PolyMem® made easy. Wounds International. Available online: www.woundsinternational.com/resources/details/polymem-dressings-made-easy
Denyer J, Pillay E, Clapham J (2017) Best practice guidelines for skin and wound care in epidermolysis bullosa. An International consensus. Wounds International. Available online: www.woundsinternational.com/uploads/resources/79912622fffa0956d1619feb123f35ed.pdf
Edwards J, Mason S (2010) An evaluation of the use of PolyMem® Silver in burn management. J Community Nurs 24(6): 16–19
Gefen A (2018) Managing inflammation by means of polymeric membrane dressings in pressure ulcer prevention. Wounds International 9(1): 250-256
Hampton S (2015) Selecting wound dressings for optimum healing. Nurs Times [online] 111(49/50): 20-23
Hegarty F, Wong M (2014) Polymeric membrane dressing for radiotherapy-induced skin reactions. Br J Nurs 23(Suppl20): S38-S46
Kahn AR (2000) A superficial cutaneous dressing inhibits inflammation and swelling in deep tissues. Pain Med 1(2): 187
Scott A (2014) Polymeric membrane dressing for radiotherapy-induced skin damage. Br J Nurs 23(10): S24-S30
Benskin LL (2016) Polymeric membrane dressings for topical wound management of patients with infected wounds in a challenging environment: A protocol with 3 case examples. Ostomy Wound Manage 62(5): 42–50
Bode C, Woodman H (2008) Two novel treatments for the prevention and treatment of radiation induced moist desquamation. Poster presentation
Burd A, Kwok CH, Hung SC, et al (2007) A comparative study of the cytotoxicity of silver based dressings in monolayer cell, tissue explant, and animal models. Wound Repair Regen 15(1): 94-104
Cutting K, Gefen A (2019) PolyMem® and countering inflammation made easy. Wounds International. Available online: www.woundsinternational.com
Cutting KF, Vowden P, and Wiegand C (2015) Wound inflammation and the role of a multifunctional polymeric dressing. Wounds International 6(2): 41–6
De Leon J, Bohn GA, DiDomenico L, et al (2016) Wound care centers: Critical thinking and treatment strategies for wounds. WOUNDS 28(10): 3-24
Denyer J (2015) Six years’ experience of PolyMem® dressings used on children with epidermolysis bullosa (EB). Poster presentation. DEBRA International conference, 24 - 26 September. London, UK
Denyer J, Agathangelou C, White R, Ousey K, HariKrishna R (2015) PolyMem® made easy. Wounds International. Available online: www.woundsinternational.com/resources/details/polymem-dressings-made-easy
Denyer J, Pillay E, Clapham J (2017) Best practice guidelines for skin and wound care in epidermolysis bullosa. An International consensus. Wounds International. Available online: www.woundsinternational.com/uploads/resources/79912622fffa0956d1619feb123f35ed.pdf
Edwards J, Mason S (2010) An evaluation of the use of PolyMem® Silver in burn management. J Community Nurs 24(6): 16–19
Gefen A (2018) Managing inflammation by means of polymeric membrane dressings in pressure ulcer prevention. Wounds International 9(1): 250-256
Hampton S (2015) Selecting wound dressings for optimum healing. Nurs Times [online] 111(49/50): 20-23
Hegarty F, Wong M (2014) Polymeric membrane dressing for radiotherapy-induced skin reactions. Br J Nurs 23(Suppl20): S38-S46
Kahn AR (2000) A superficial cutaneous dressing inhibits inflammation and swelling in deep tissues. Pain Med 1(2): 187
Scott A (2014) Polymeric membrane dressing for radiotherapy-induced skin damage. Br J Nurs 23(10): S24-S30



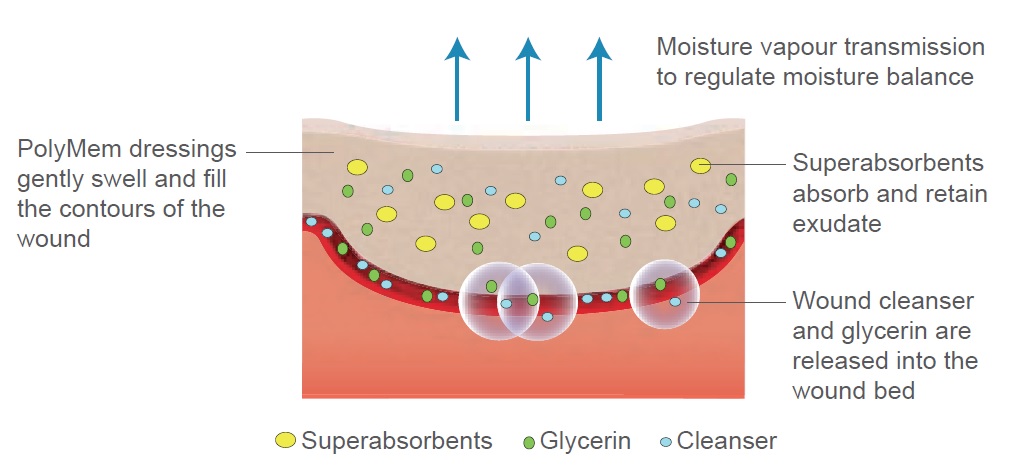 Figure 1. The structure and function of PolyMem®.
Figure 1. The structure and function of PolyMem®.