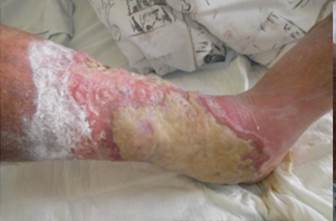
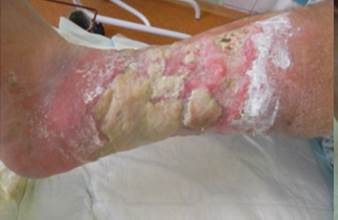
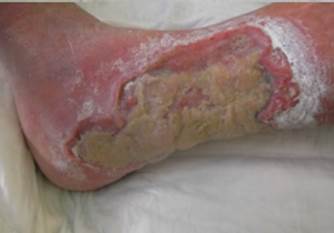
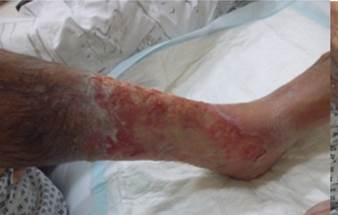
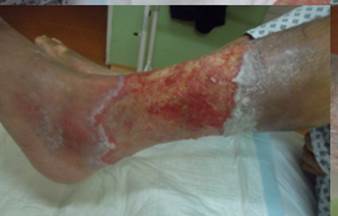
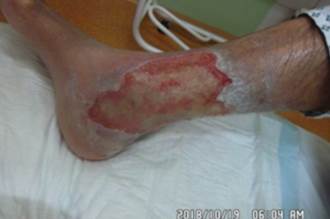
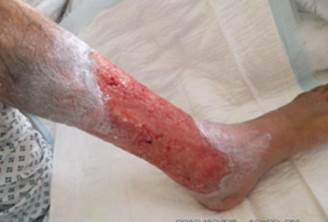
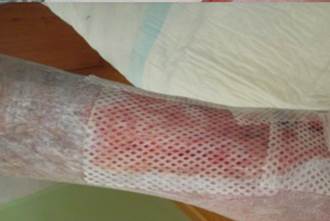
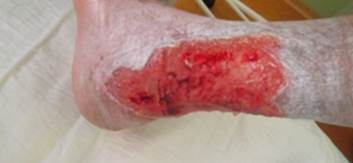
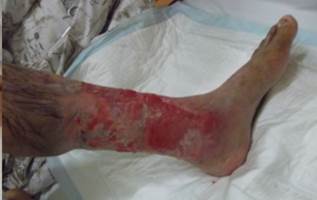
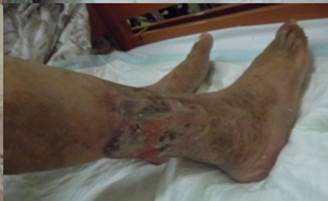
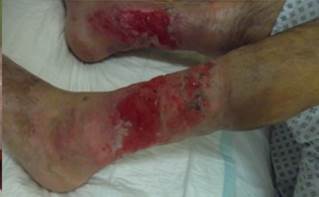
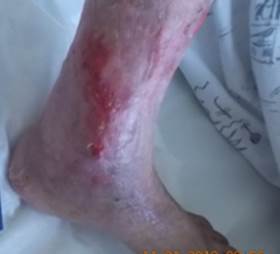
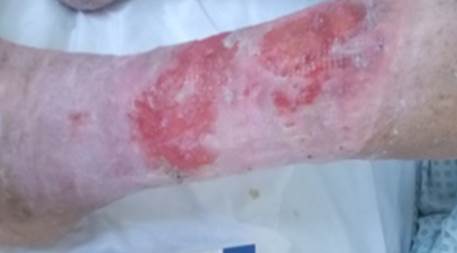
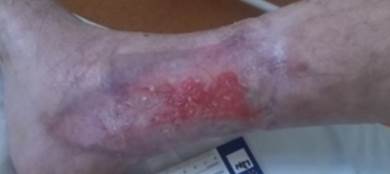
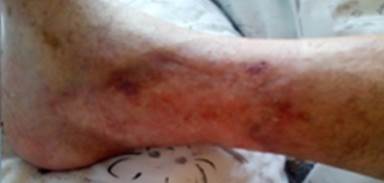
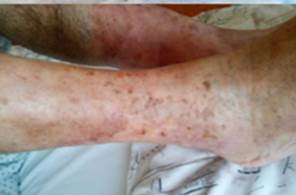
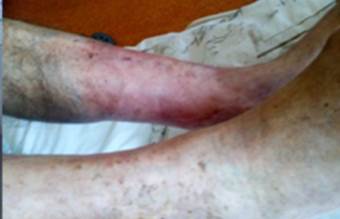
References
Agale SV (2013) Chronic leg ulcers: epidemiology, aetiopathogenesis, and management. Ulcers 413604, doi:10.1155/2013/413604
Alam F, Islam MA, Gan SH, Khalil MI (2014) Honey: a potential therapeutic agent for managing diabetic wounds. Evid Based Complement Alternat Med, 2014, 169130, doi:10.1155/2014/169130
Chaplin J (2004) Wound management in palliative care. Nurs Stand 19: 39–42, doi:10.7748/ns2004.09.19.1.39.c3693
Dale B, Emmons KR (2014) Palliative wound care: principles of care. Home Healthc Nurse 32: 48–53: quiz 54-45, doi:10.1097/NHH.0000000000000004
Farage MA, Miller KW, Elsner P, Maibach HI (2013) Characteristics of the Aging Skin. Adv Wound Care (New Rochelle) 2: 5–10, doi:10.1089/wound.2011.0356
Frykberg RG, Banks J (2015) Challenges in the Treatment of Chronic Wounds. Adv Wound Care (New Rochelle) 4: 560–82, doi:10.1089/wound.2015.0635.
Grocott P, Gray D (2010) The argument for palliative wound care. Wounds UK 6: 167–8
Jaul E, Barron J, Rosenzweig JP, Menczel J (2018) An overview of co-morbidities and the development of pressure ulcers among older adults. BMC Geriatr 18: 305, doi:10.1186/s12877-018-0997-7
Kelechi T, Prentice M, Madisetti M, Brunette G, Mueller M (2017) Palliative Care in the Management of Pain, Odor, and Exudate in Chronic Wounds at the End of Life: A Cohort Study. Journal of Hospice & Palliative Nursing 19: 17–25, doi:10.1097/NJH.0000000000000306
Mekkes JR, Loots MA, Van Der Wal AC, Bos JD (2003) Causes, investigation and treatment of leg ulceration. Br J Dermatol 148: 388-401, doi:10.1046/j.1365-2133.2003.05222.x
Nair HKR, Tatavilis N, Pospisilova I, Kucerov J, Cremers NAJ (2020) Medical-Grade Honey Kills Antibiotic-Resistant Bacteria and Prevents Amputation in Diabetics with Infected Ulcers: A Prospective Case Series. Antibiotics (Basel) 9: doi:10.3390/antibiotics9090529.
Pleeging CCF, Coenye T, Mossialos D et al (2020) Synergistic Antimicrobial Activity of Supplemented Medical-Grade Honey against Pseudomonas aeruginosa Biofilm Formation and Eradication. Antibiotics 9: 866, doi:doi:10.3390/antibiotics9120866.
Sgonc R, Gruber, J (2013) Age-related aspects of cutaneous wound healing: a mini-review. Gerontology 59: 159–64, doi:10.1159/000342344
Smaropoulos E, Cremers NAJ (2019) The pro-healing effects of medical grade honey supported by a pediatric case series. Complement Ther Med 45: 14–18, doi:10.1016/j.ctim.2019.05.014.
Smaropoulos E, Cremers NA (2020) Medical grade honey for the treatment of paediatric abdominal wounds: a case series. J Wound Care 29: 94–99, doi:10.12968/jowc.2020.29.2.94.
Smaropoulos E, Cremers NAJ (2020) Treating severe wounds in pediatrics with medical grade honey: A case series. Clin Case Rep 8: 469–76, doi:10.1002/ccr3.2691
Smaropoulos E, Cremers NAJ (2020) Medical grade honey for the treatment of extravasation-induced injuries in preterm neonates – a case series. Advances in Neonatal Care 21(2): 122–32
Yapucu Gunes U, Eser I (2007) Effectiveness of a honey dressing for healing pressure ulcers. J Wound Ostomy Continence Nurs 34: 184–90, doi:10.1097/01.WON.0000264833.11108.35
Alam F, Islam MA, Gan SH, Khalil MI (2014) Honey: a potential therapeutic agent for managing diabetic wounds. Evid Based Complement Alternat Med, 2014, 169130, doi:10.1155/2014/169130
Chaplin J (2004) Wound management in palliative care. Nurs Stand 19: 39–42, doi:10.7748/ns2004.09.19.1.39.c3693
Dale B, Emmons KR (2014) Palliative wound care: principles of care. Home Healthc Nurse 32: 48–53: quiz 54-45, doi:10.1097/NHH.0000000000000004
Farage MA, Miller KW, Elsner P, Maibach HI (2013) Characteristics of the Aging Skin. Adv Wound Care (New Rochelle) 2: 5–10, doi:10.1089/wound.2011.0356
Frykberg RG, Banks J (2015) Challenges in the Treatment of Chronic Wounds. Adv Wound Care (New Rochelle) 4: 560–82, doi:10.1089/wound.2015.0635.
Grocott P, Gray D (2010) The argument for palliative wound care. Wounds UK 6: 167–8
Jaul E, Barron J, Rosenzweig JP, Menczel J (2018) An overview of co-morbidities and the development of pressure ulcers among older adults. BMC Geriatr 18: 305, doi:10.1186/s12877-018-0997-7
Kelechi T, Prentice M, Madisetti M, Brunette G, Mueller M (2017) Palliative Care in the Management of Pain, Odor, and Exudate in Chronic Wounds at the End of Life: A Cohort Study. Journal of Hospice & Palliative Nursing 19: 17–25, doi:10.1097/NJH.0000000000000306
Mekkes JR, Loots MA, Van Der Wal AC, Bos JD (2003) Causes, investigation and treatment of leg ulceration. Br J Dermatol 148: 388-401, doi:10.1046/j.1365-2133.2003.05222.x
Nair HKR, Tatavilis N, Pospisilova I, Kucerov J, Cremers NAJ (2020) Medical-Grade Honey Kills Antibiotic-Resistant Bacteria and Prevents Amputation in Diabetics with Infected Ulcers: A Prospective Case Series. Antibiotics (Basel) 9: doi:10.3390/antibiotics9090529.
Pleeging CCF, Coenye T, Mossialos D et al (2020) Synergistic Antimicrobial Activity of Supplemented Medical-Grade Honey against Pseudomonas aeruginosa Biofilm Formation and Eradication. Antibiotics 9: 866, doi:doi:10.3390/antibiotics9120866.
Sgonc R, Gruber, J (2013) Age-related aspects of cutaneous wound healing: a mini-review. Gerontology 59: 159–64, doi:10.1159/000342344
Smaropoulos E, Cremers NAJ (2019) The pro-healing effects of medical grade honey supported by a pediatric case series. Complement Ther Med 45: 14–18, doi:10.1016/j.ctim.2019.05.014.
Smaropoulos E, Cremers NA (2020) Medical grade honey for the treatment of paediatric abdominal wounds: a case series. J Wound Care 29: 94–99, doi:10.12968/jowc.2020.29.2.94.
Smaropoulos E, Cremers NAJ (2020) Treating severe wounds in pediatrics with medical grade honey: A case series. Clin Case Rep 8: 469–76, doi:10.1002/ccr3.2691
Smaropoulos E, Cremers NAJ (2020) Medical grade honey for the treatment of extravasation-induced injuries in preterm neonates – a case series. Advances in Neonatal Care 21(2): 122–32
Yapucu Gunes U, Eser I (2007) Effectiveness of a honey dressing for healing pressure ulcers. J Wound Ostomy Continence Nurs 34: 184–90, doi:10.1097/01.WON.0000264833.11108.35