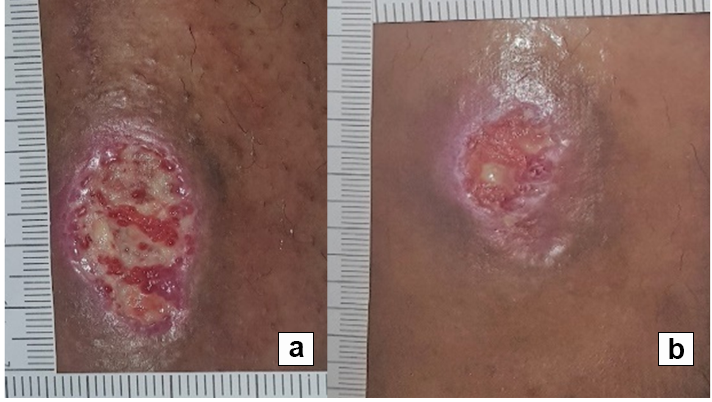
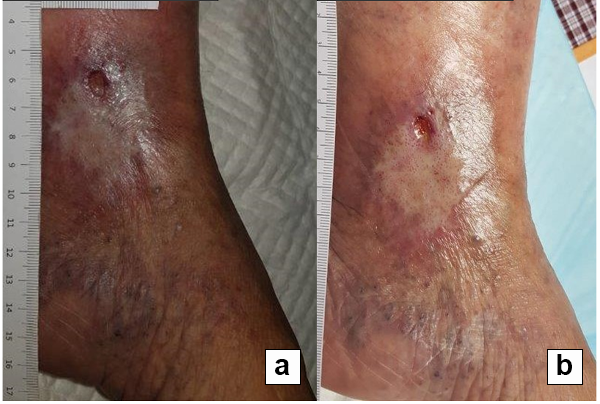
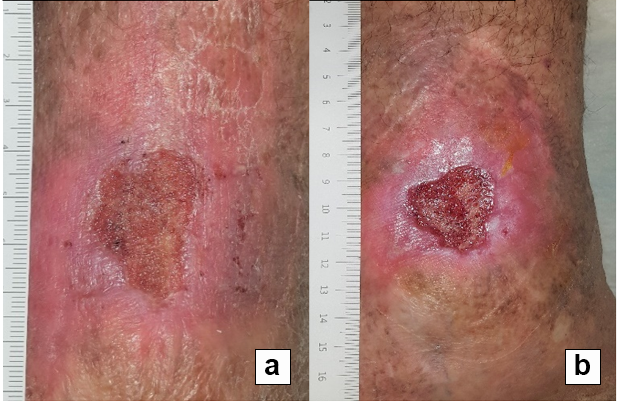
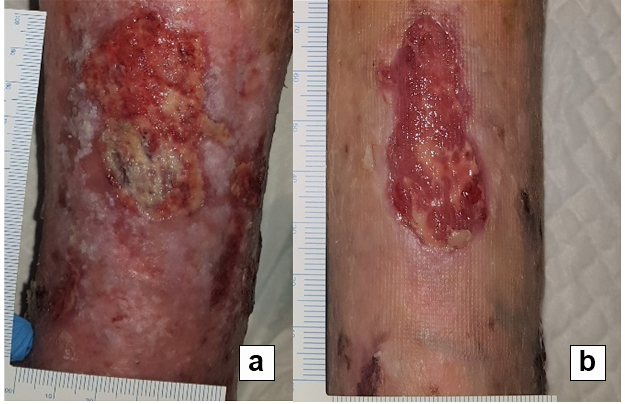
References
Atkin L (2014) Understanding methods of wound debridement. Br J Nurs 23(12): S10–12, S14–15
Cremers N, Belas A, Santos Costa S, Couto I, de Rooster H, Pomba C (2020) In vitro antimicrobial efficacy of two medical grade honey formulations against common high-risk meticillin-resistant staphylococci and Pseudomonas spp. pathogens. Vet Dermatol 31(2): 90–96
Diegelmann RF, Evans MC (2004) Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 9: 283–9
Erfurt-Berge C, Renner R (2014) Recent developments in topical wound therapy: impact of antimicrobiological changes and rebalancing the wound milieu. Biomed Res Int 2014; ID: 819525
Eming SA, Martin P, Tomic-Canic M (2014) Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med 6(265): 265sr6
Gulati S, Qureshi A, Srivastava A, Kataria K, Kumar P, Ji AB (2014) A Prospective Randomized Study to Compare the Effectiveness of Honey Dressing vs. Povidone Iodine Dressing in Chronic Wound Healing. Indian J Surg 76(3):193–8
Hermanns R, Mateescu C, Thrasyvoulou A, Tananaki C, Wagener FADTG, Cremers NAJ (2020) Defining the standards for medical grade honey. Journal of Apicultural Research 59(2): 125–35
International consensus (2011) The role of proteases in wound diagnosis. An Expert working group review. Wounds International 1–13
Martinotti S, Bucekova M, Majtan J, Ranzato E (2018) Honey: an effective regenerative medicine product in wound management. Curr Med Chem 26(27): 5230–40
McCarty SM, Percival SL (2013) Proteases and Delayed Wound Healing. Adv Wound Care (New Rochelle) 2(8): 438–47
Molan P, Rhodes T (2015)Honey: A Biologic Wound Dressing. Wounds 27(6): 141–51 Rundhaug JE (2005) Matrix metalloproteinases and angiogenesis. J Cell Mol Med 9(2): 267–85
Simon A, Traynor K, Santos K, Blaser G, Bode U, Molan P (2009) Medical honey for wound care-still the 'latest resort'? Evid Based Complement Alternat Med 6(2): 165–73
Smaropoulos E, Cremers NA (2020a) Treating severe wounds in pediatrics with medical grade honey: A case series. Clinical Case Reports. 8(3): 469–76
Smaropoulos E, Cremers NA (2020b) Medical grade honey for the treatment of paediatric abdominal wounds: a case series. J Wound Care 29(2): 94–9
Smaropoulos E, Cremers NA (2021) Medical-Grade Honey for the Treatment of Extravasation-Induced Injuries in Preterm Neonates: A Case Series. Adv Neonatal Care 21(2):122-132. DOI: 10.1097/ANC.0000000000000781
Smeets R, Ulrich D, Unglaub F, Woltje M, Pallua N (2008) Effect of oxidised regenerated cellulose/collagen matrix on proteases in wound exudate of patients with chronic venous ulceration. Int Wound J 5(2): 195–203
Westby MJ, Norman G, Dumville JC, Stubbs N, Cullum N (2016) Protease-modulating matrix treatments for healing venous leg ulcers. Cochrane Database Syst Rev. 12: CD011918
Wild T, Rahbarnia A, Kellner M, Sobotka L, Eberlein T (2010) Basics in nutrition and wound healing. Nutrition 26(9): 862–6
Yaghoobi R, Kazerouni A, Kazerouni O (2013) Evidence for Clinical Use of Honey in Wound Healing as an Anti-bacterial, Anti-inflammatory Anti-oxidant and Anti-viral Agent: A Review. Jundishapur J Nat Pharm Prod 8(3): 100–104
Yilmaz AC, Aygin D (2020) Honey Dressing in Wound Treatment: A Systematic Review. Complement Ther Med 51: 102388; doi: 10.1016/j.ctim.2020.102388
Cremers N, Belas A, Santos Costa S, Couto I, de Rooster H, Pomba C (2020) In vitro antimicrobial efficacy of two medical grade honey formulations against common high-risk meticillin-resistant staphylococci and Pseudomonas spp. pathogens. Vet Dermatol 31(2): 90–96
Diegelmann RF, Evans MC (2004) Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 9: 283–9
Erfurt-Berge C, Renner R (2014) Recent developments in topical wound therapy: impact of antimicrobiological changes and rebalancing the wound milieu. Biomed Res Int 2014; ID: 819525
Eming SA, Martin P, Tomic-Canic M (2014) Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med 6(265): 265sr6
Gulati S, Qureshi A, Srivastava A, Kataria K, Kumar P, Ji AB (2014) A Prospective Randomized Study to Compare the Effectiveness of Honey Dressing vs. Povidone Iodine Dressing in Chronic Wound Healing. Indian J Surg 76(3):193–8
Hermanns R, Mateescu C, Thrasyvoulou A, Tananaki C, Wagener FADTG, Cremers NAJ (2020) Defining the standards for medical grade honey. Journal of Apicultural Research 59(2): 125–35
International consensus (2011) The role of proteases in wound diagnosis. An Expert working group review. Wounds International 1–13
Martinotti S, Bucekova M, Majtan J, Ranzato E (2018) Honey: an effective regenerative medicine product in wound management. Curr Med Chem 26(27): 5230–40
McCarty SM, Percival SL (2013) Proteases and Delayed Wound Healing. Adv Wound Care (New Rochelle) 2(8): 438–47
Molan P, Rhodes T (2015)Honey: A Biologic Wound Dressing. Wounds 27(6): 141–51 Rundhaug JE (2005) Matrix metalloproteinases and angiogenesis. J Cell Mol Med 9(2): 267–85
Simon A, Traynor K, Santos K, Blaser G, Bode U, Molan P (2009) Medical honey for wound care-still the 'latest resort'? Evid Based Complement Alternat Med 6(2): 165–73
Smaropoulos E, Cremers NA (2020a) Treating severe wounds in pediatrics with medical grade honey: A case series. Clinical Case Reports. 8(3): 469–76
Smaropoulos E, Cremers NA (2020b) Medical grade honey for the treatment of paediatric abdominal wounds: a case series. J Wound Care 29(2): 94–9
Smaropoulos E, Cremers NA (2021) Medical-Grade Honey for the Treatment of Extravasation-Induced Injuries in Preterm Neonates: A Case Series. Adv Neonatal Care 21(2):122-132. DOI: 10.1097/ANC.0000000000000781
Smeets R, Ulrich D, Unglaub F, Woltje M, Pallua N (2008) Effect of oxidised regenerated cellulose/collagen matrix on proteases in wound exudate of patients with chronic venous ulceration. Int Wound J 5(2): 195–203
Westby MJ, Norman G, Dumville JC, Stubbs N, Cullum N (2016) Protease-modulating matrix treatments for healing venous leg ulcers. Cochrane Database Syst Rev. 12: CD011918
Wild T, Rahbarnia A, Kellner M, Sobotka L, Eberlein T (2010) Basics in nutrition and wound healing. Nutrition 26(9): 862–6
Yaghoobi R, Kazerouni A, Kazerouni O (2013) Evidence for Clinical Use of Honey in Wound Healing as an Anti-bacterial, Anti-inflammatory Anti-oxidant and Anti-viral Agent: A Review. Jundishapur J Nat Pharm Prod 8(3): 100–104
Yilmaz AC, Aygin D (2020) Honey Dressing in Wound Treatment: A Systematic Review. Complement Ther Med 51: 102388; doi: 10.1016/j.ctim.2020.102388