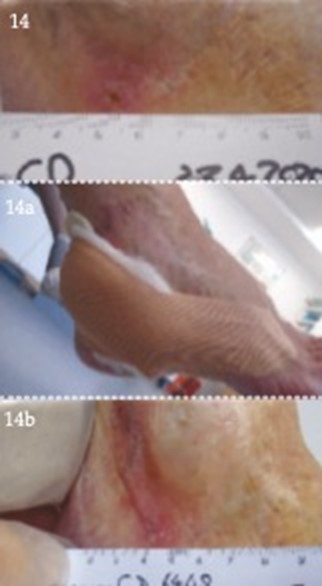
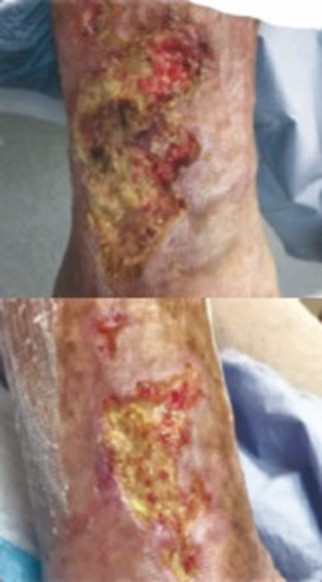
References
Artlett C M (2012) Inflammasomes in wound healing and fibrosis . J Pathol 229(2): 157–67
Bertelli DF, De Oliver P, Gimenes AS, Morena MA (2013) Postural drainage and manual lymphatic drainage for lower limb oedema in women with morbid obesity after bariatric surgery: a randomised control trial. Am J Phys Med Rehabil 92(8): 697–703
Bjork R (2013) Positive Stemmer’s sign yields a definitive lymphedema diagnosis in 10 seconds or less. Wound Care Advisor 2( 2): 10–14
Bjork R, Hettrick H (2018) Endothelial glycocalyx layer and interdependence of lymphatic and integumentary systems. Wounds Int 9(2): 50–5
Blanco EG, Gonzalez MS (2020) The efficiacy of kinesio taping in the treatment of lymphoedema after breast cancer: a systematic review. J Lymphoedema 15(1): 71–6
Burnand KG, Whimster I, Naidoo A, Browse NL (1982) Pericapillary fibrin in the ulcer-bearing skin of the leg: The cause of lipodermatosclerosis and venous ulceration. Br Med J (Clin Res Ed) 285: 1071–2
Carati CJ, Anderson SN, Gannon BJ, Piller N (2003) Treatment of post mastectomy lymphoedema with low level laser therapy. Cancer 98: 1114–22
Charles H (2103) Chronic oedema, compression therapy and static stiffness index . Wounds UK 9(2) Supplement 2: 4–10
Dyson M, Lyder C (2001) In: Morison MJ, ed. The prevention and treatment of pressure ulcers. Mosby, Edinburgh: 177–93
Ellis M (2015) Lymphovenous disease and its effects on the lower limb. J Community Nurs 29(1): 32–40
Eliska O, Eliskova M (1997) Laser and Lymphatic. Proceedings of 16th ISL congress. Lymphology: 474–7
Elwell R (2015) Compression bandaging for chronic oedema: applying science to reality. Br J Community Nurs 20(5 Suppl): S4–7
European Wound Management Association (2005) Focus Document: Lymphoedema bandage in practice. London: MEP Ltd
Farrow W (2010) Phlebolymphedema – a common underdiagnosed and undertreated problem in the wound care clinic. J Am Col Certif Wound Spec 2(1): 14–23
Finlayson K, Miaskowski C, Alexander K, Lui WH, Aouizerat B, parker C ,Pennis DM , Edwards D (2017) Distinct wound healing and quality of life outcomes in subgroups of patients with venous legs with different cluster experiences. J Pain Symptom Manage 53(5): 871–9
Földi E, Sauerwald A, Hennig B (2000) Effect of complex decongestive physiotherapy on gene expression for the inflammatory response in peripheral lymphedema. Lymphology 33(1): 19–23
Green T (2007) Leg ulcer management in patients with chronic oedema. Wound Essentials 2: 46–58
Guest JF, Ayoub N, McIlwraith T, et al (2015) Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open 5(12): e009283
Guest JF, Fuller GW, Vowden P (2020) Cohort study evaluating the burden of wounds to the UK’s National Health Service in 2017/2018: update from 2012/2013 BMJ. BMJ Open 10: e045253
Harding K, et al (2015) Simplifying venous leg ulcer management. Consensus recommendations. Wounds International. Available online: www.woundsinternational.com
Hodgson P, Letellier ME, Schumann LH (2011) Use of tissue mobilising compression system. J Lymphoedema 6(2): 91–3
Hopkins A, Worboys F, Bull R, et al (2011) Compression strapping: the development of a novel compression technique to enhance compression therapy and healing for ‘hard-to-heal’ leg ulcers. Inter Wound J 8(5): 474–83
Hopkins A, Worboys F, Partsch H (2013) The use of strapping to increase local pressure: reporting of a sub-bandage pressure study. Veins Lymphatics 2(1): 12
Kozanoglu E, Basaran S, Paydas S, Sarpel T (2009) Efficacy of pneumatic compression and low-level laser therapy in the treatment of postmastectomy lymphoedema: a randomized controlled trial. Clin Rehabil 23(2): 117–24
Macmillan Cancer Suport (2011) Specialist lymphoedema services: An evidence review. Available online: www.Macmillan.org.uk/servicesimpact
Minatel DG, Frade MA, Franca SC, Enwemeka CS (2009) Phototherapy promotes healing of chronic diabetic leg ulcers that failed to respond to other therapies. Lasers Surg Med 41(6): 433–41
Moffatt CJ, Morgan P, Doherty D (2005) The Lymphoedema Framework: a consensus on lymphoedema bandaging. In: European Wound Management Association (EWMA) Focus Document: Lymphoedema bandaging in practice. MEP Ltd, London
Morgan PA, Moody M, Franks PJ, Moffatt CJ, Doherty DC (2005) Assessing community nurses’ level of knowledge of lymphoedema. Br J Nurs 14(1): 8–13
Mortimer P, Browse N (2003) The relationship between the lymphatics and chronic venous disease. In: Browse N, Burnand KG, Mortimer PS (2003) Diseases of the Lymphatics. Arnold, London: 293–6
Mosti G, Cavezzi A, Partsch H, Urso S, Campana F (2015) Adjustable Velcro wrap devices are more effective than inelastic compression bandages in reducing oedema in the initial treatment phase: a randomised control trial. Eur Vas Endovasc Surg 50(3): 368–74
Omar MT, Shaheen AA, Zafar H (2012) A systematic review of the effect of low-level laser therapy in the management of breast cancer-related lymphedema. Support Care Cancer 20(11): 2977–84
Partsch H, Mortimer P (2015) Compression for leg wounds. Br J Dermatol 173(2): 359–69
Piller N, Thelander A (1998) Treating chronic post mastectomy lymphoedema with low level laser therapy: a cost effective strategy to reduce lymphoedema severity and improve the quality of patient life. Lymphology 31(2): 74–86
Schubert V (2001) Effects of phototherapy on pressure ulcer healing in elderly patients after a falling trauma. A prospective, randomized, controlled study. Photodermatol Photoimmunol Photomed 17(1): 32–8
Stanton J (2020) Development of the hybrid tissue viability nurse/lymphoedema nurse. J Community Nurs 34(4): 46–51
Todd M (2019) Chronic oedema: impact and management. Br J Nurs 22(11): 623–7
White R, Price P, Whittaker J, Williams SA (2014) Lymphovenous oedema (phlebolymphoedema): The nature and extent of the problem. Wounds UK 10(1): 22–8
Whittaker J Williams A, Pope D, et al (2015) Clinical audit of a lymphoedema bandaging system: a foam roll and cohesive short stretch bandages. J Wound Care 24(3): 83–4; 86–90; 92–4
Wigg J (2009) Use and response to treatment using low level laser therapy. J Lymphoedema 4(2): 73–6
Wigg J (2015) The Complete Package, Lymphoedema Management (FG-MLD® training), training manual. Lymphoedema Training academy
Wigg J (2016) Fluoroscopy guided manual lymphatic drainage from research to the classroom. International Lymphoedema Framework Conference/Australasian Lymphology Association Conference
Williams A (2003) An overview of non-cancer related chronic oedema — a UK perspective. Worldwide Wounds. Available online: www.worldwidewounds.com/2003/april/Williams/Chronic-Oedema.html
Williams A (2009) Chronic oedema in patients with CVI and ulceration of the lower limb. Br J Community Nurs 14(10): S4–8
Wound Care People (2019) Best practice in the community. Chronic oedema. Wound Care People, Wixford
Wounds UK (2019) Best Practice Statement: Addressing complexities in the management of venous leg ulcers. London: Wounds UK. Available to download from: www.wounds-uk.com



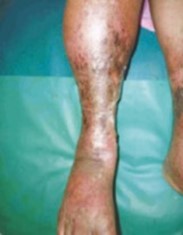
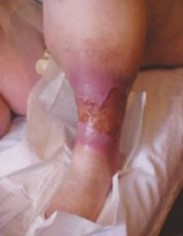

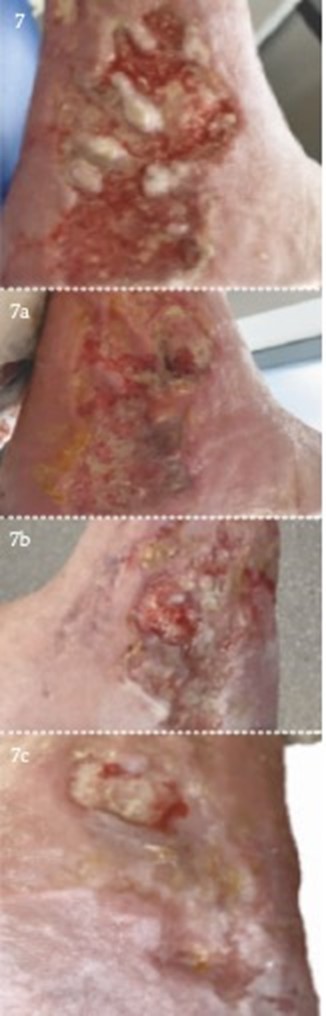
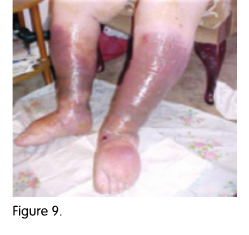
.png)