References
Chadwick P, McCardle J (2016) Open, non-comparative, multicentre post clinical study of the performance and safety of a gelling fibre wound dressing on diabetic foot ulcers. J Wound Care 25(5): 290–300
Chaloner D, Poole M (1995) Cavity wound management in the community. Br J Nurs 4(10): 556–61
Charlesworth B, Pilling C, Chadwick P, Butcher M (2014) Dressing-related trauma: clinical sequelae and resource utilization in a UK setting. Clinicoeconomics and Outcomes Research 6: 227–39
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4010615/
Cooper P (2006) How to probe a wound during assessment to help determine treatment options. Wound Essentials (1): 87–9
European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance (2019) Prevention and treatment of pressure ulcers/injuries: Quick reference guide. Emily Haesler (Ed.). EPUAP/NPIAP/PPPIA, 2019
EWMA (2007) Position document: Topical negative pressure in wound management. MEP Ltd, London. Available online: www.ewma.org
Jones J (2006) The use of gauze: will it ever change? Int Wound J 3(2): 79–88
Guest JF, Ayoub N, Mcllwraith T et al (2015) Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open 5(12): e009283
Lindholm C, Bergsten A, Berglund E (1999) Chronic wounds and nursing care. J Wound Care 8(1): 5–10
Maddineni NK, Kodura SK, Surath S et al (2015) Negative pressure wound therapy in orthopaedic post-operative infections. Journal of NTR University Health Sciences 4(4): 257–62
Mahoney K (2020) Part 1: Wound assessment. J Comm Nurs 34(2): 28–35
Metcalf DG, Bowler PG, Hurlow J (2014) A clinical algorithm for wound biofilm identification. J Wound Care 23(3): 137–42
Morgan-Jones R, Bishay M, Hernández-Hermosa, JA, et al (2019) Consensus meeting report. Incision care and dressing selection in surgical wounds: Findings from an international meeting of surgeons. Wounds International, London. Available online: www.woundsinternational.com
Ousey K, O’Connor L, Doughty D, et al (2017) IAD Made Easy. Wound Int: 1–6
Phillips CJ, Humphreys I, Fletcher J et al (2015) Estimating the costs associated with the management of patients with chronic wounds using linked routine data. Int Wound J doi:10.1111/iwj.12443
Quain AM and Khardori NM (2015) Nutrition in Wound care Management: A Comprehensive overview. Wounds 27(12): 327–35
Skerritt L and Moore Z (2014) The prevalence, aetiology and management of wounds in a community care area in Ireland. Community Wound Care 19 (Sup6): S11–S17
Smet S (2015) An open, non-comparative, multicentre clinical investigation to evaluate the performance and safety of a gelling fibre dressing for the treatment of pressure ulcers. Poster presentation. European Pressure Ulcer Advisory Panel Conference. Gent, Belgium
Smith N, Overland J, Greenwood J (2015) Local management of deep cavity wounds – current and emerging therapies. Chronic Wound Care Management and Research (2): 159–70
Srinivasaiah N, Dugdall H, Barrett S et al (2007) A point prevalence survey of wounds in North east England. J Wound Care 16(10): 413–19
Timmons J, Cooper P (2008) How to systemically assess a patient with a cavity wound. Wounds UK Supplement (4)2: 4–10
Timmons J, Dugid K, Pirie G et al (2008) The management of a patient with a cavity wound. Wounds UK Supplement (4)2: 11–18
Vowden K (2016) Defining, assessing and managing cavity wounds. Wounds UK 12(1): 18–23
Vowden K, Vowden P (2014) Wound dressings: principles and practice, Surgery (Oxford) 35(9): 489–94
Williams C (1997) Treatment of cavity wounds. Practice Nursing 8(13): 31–33
White R (2005) Evidence for atraumatic soft silicone wound dressing use. Wounds UK 1(3): 104–9
White R (2008) A multinational survey of the assessment of pain when removing dressings. Wounds UK 4(1): 14–22
Wounds UK (2016) Quick Guide Cavity Wounds. Wounds UK, London. Available online: www.wounds-uk.com
Wounds UK (2018) Best practice statement: Improving holistic assessment of chronic wounds. Wounds UK, London. Available online: www.wounds-uk.com
WUWHS (2018) Consensus document. Surgical wound dehiscence: improving prevention and outcomes. Wounds International, London. Available online: www.woundsinternational.com
WUWHS (2019a) Consensus document. Wound exudate: effective assessment and management. Wounds International, London. Available online: www.woundsinternational.com
WUWHS (2019b) Consensus document. Surgical wound dehiscence: Improving prevention and outcomes. Wounds International, London. Available online: www.woundsinternational.com
Young T (2017) Back to basics: understanding moisture-associated skin damage. Wounds UK 13(2): 56–65



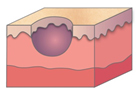
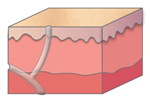
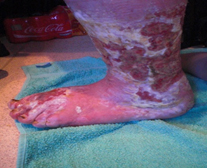
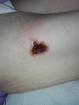
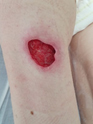
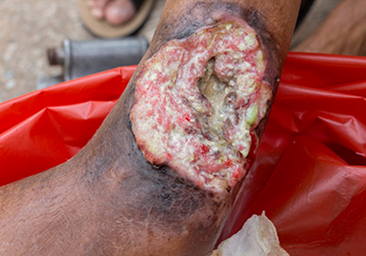 Figure 1. A malignant cavity wound.
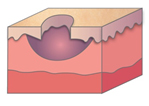
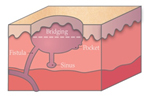
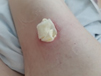
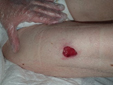
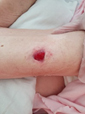
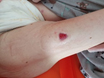
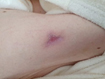
Figure 1. A malignant cavity wound.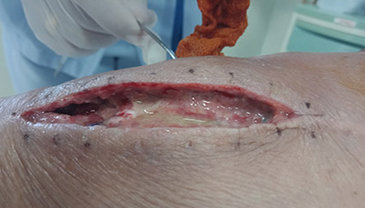 Figure 2. A dehisced surgical cavity wound.
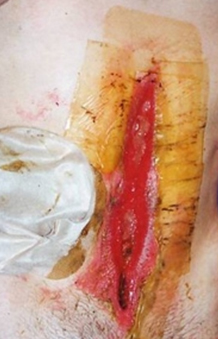
Figure 2. A dehisced surgical cavity wound.